Infant formula manufacturing process pdf
2 Understanding the history and structure of WIC’s competitive bidding process for infant formula — as well as the resulting federal savings — can help policymakers ensure that this critical
Current Good Manufacturing Practice, Quality Control Procedures, Quality Factors, Notification Requirements, and Records and Reports, for the Production of Infant Formula…
Following consultation, the revised Animal Products Notice: Manufacture of Dairy Based Infant Formula Products and Formulated Supplementary Foods for Young Children has been published. A summary of the submissions received during the consultation is also available. Animal Products Notice
Several aspects must be taken into consideration when designing a new infant formula production line. When dealing with process plants for the baby food industry, safety and reliability is the most important focus point for a process plant supplier.
19/12/2017 · The new infant formula submission must include: 1) the product name and a description of the physical form, 2) an explanation of why it is a new infant formula, 3) …
2/07/2015 · Powdered infant formula contamination can occur during the remaining stages in the production process, especially during phases close to the packing of the final product (30, 31). Proudy et al. found that the majority (78%) of isolates were recovered from processing areas (surfaces around dryers and blenders); 12% from ingredients and 10% from final product ( 31 ).
Application Our best-practice line for continuous production of powdered infant formula. Highlights • greater convenience and lower environmental impact.
If a manufacturing premises is only packing finished infant formula, then both the packing premises and the premises that produced and supply the finished bulk product should fill in …
Depending on the raw materials used and the manufacturing process, the shelf life of liquid infant formula ranges from 6 to 12 months. A longer shelf life can be achieved by using packaging containers with good oxygen barrier properties and small and/or gas flushed head spaces.
Update on China infant formula registration process The

China clarifies infant formula plans The Australian
Infant Formula Processing There are many stages in a complete process line for producing infant formula and consideration needs to be taken for the type of ingredients, their quality, and their application (liquid, powder in wet or dry compounding).
infant formula and Codex Code of Hygienic practice for Infant Formula, CAC/RCP 66-2008 to minimise contamination risks – UNICEF and MSF currently collaborate in audits on TM suppliers in order to identify and address quality issues in production.
3/03/2012 · Golden Fern Infant Formula is made and packed in New Zealand under strict quality guidelines. This video shows the production process. All Golden Fern products are …
Araçatuba (São Paulo), 28 September 2007 – Today, Nestlé opened the world’s most modern infant formula plant in Araçatuba, Brazil. The plant will produce Nestlé’s latest infant formulae NAN Pro 1, NAN Pro 2, Nestogeno 1, Nestogeno 2, Nestogeno Plus, Nidex, and NAN AR.

Cowala Infant Formula Stage 1 is an unique formulation, specially designed by Australian and New Zealand scientists as per strict Australian New Zealand standards to meet the needs of bottle fed infants. This product is processed and packed in New Zealand by HACCP and Good Manufacturing Practices “GMP” certified factory.
Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate a suitable protein source for infant formula production. Keywords: Pisum sativum; oligosaccharides; inositol phosphate; phytate; phytase INTRODUCTION Pea is an important grain legume, as both human food and animal feed. A valuable part of the pea is the protein fraction, which …
in the form of a specific matrix (infant formula) that could be the only source of feeding of an infant. This document is intended for practitioners, researchers, and
Guide to good manufacturing and hygiene practices for metal packaging in contact with food 1 Guide to good manufacturing and hygiene practices for metal packaging in contact with food . Contents Preface 1 1 Purpose and scope 5 1.1 Introduction 6 1.2 Scope 6 1.3 Light metal packaging and drums – the European market 7 2 Due diligence 8 2.1 Introduction 9 2.2 Suitability for purpose 9 2.3 Process
Higher birth rates in developing regions expected to drive the growth of the market for infant formula. Introduction. In terms of revenue, the global infant formula market is estimated to expand at a CAGR of 9.5% over the forecast period, owing to numerous factors, about which FMI offers thorough insights and forecasts in this report.
3/11/2014 · Introduction. Human milk is widely recommended as the optimal source of nutrition for infants. 1, 2 While the composition of milk changes over the course of lactation, 3 fat provides approximately half of the energy of human milk.
manufacturing process capable of exclusively streaming that milk into finished infant formula. Following the rapid growth and sustained success of The a2 Milk Company’s infant
Processing applications for baby food and infant formula. Baby food production is a rapidly developing category worldwide, characterised by strong, science-driven innovations for added value and care for babies beyond simply nutrition.
The registration will ensure that direct exports to China of a2MC’s China label infant formula products (a2 Platinum® infant formula), which currently comprise ~8% of a2MC’s total infant formula sales, can continue from 1 January 2018.
Manufacturing Infant Formula Milk Powder . For infant formula technology transfer please contact us using the contact page. Watson Dairy Consulting have significant experience in establishing manufacturing and quality systems for Infant Formula production.
The information collected on the microbiological quality of powdered infant formula will supplement the communication message on the safe handling of powdered infant formula that was developed for consumers and caregivers and is available on the NSW Food Authority website.
The Nutritional Adequacy of Infant Formula George Kent, Ph.D. Government agencies that regulate infant formula have been concerned about its safety, worrying about things like contamination with bacteria and insect parts. Questionable formula has been subject to government-ordered recalls. This attention reinforces people’s confidence that national governments are ensuring the quality of
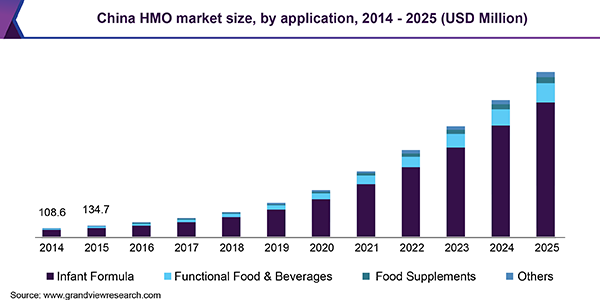
Human Milk Oligosaccharides (HMO) Market Size, Share & Trends Analysis Report By Application (Food Supplements, Functional Food & Beverages, Infant Formula), By Region, And Segment Forecasts, 2018 – 2025
China’s finance ministry said overnight that infant milk formula imports to China through international websites would need official approval by 2018.
Only a few producers of infant formula milk exploit fully their production tool, both in the matter of quality of the finished product as well as the optimization of the manufacturing process and therefore the performance output.
A NEW MODEL FOR INFANT FORMULA TESTING Infant formula manufacturing is a complex process involving global raw material supply chains. In this competitive marketplace with many variables, brand protection is of utmost
The HA infant formula is based on 100% whey protein of cow’s milk. The process involves hydrolysis of proteins with Nestlé’s proprietary predigesting whey protein process. The process involves hydrolysis of proteins with Nestlé’s proprietary predigesting whey protein process.
Safety and Quality in Infant Formula spxflow.com
to produce when it applies for the processing and production of infant formula milk powder (0-6 months, stage 1), follow on formula milk powder (6-12 months, stage 2) and growing up formula milk powder (12-36 months, stage 3) for infant & growing-ups (0-36 months) using cow milk or goat milk and their processed products (whey powder, whey protein powder, skim milk powder, whole milk powder
For example, according to 21CFR106.96, a manufacturer of an eligible infant formula must show that a formula supports infant normal physical growth by conducting, in accordance with good clinical practice, an adequate and well controlled growth monitoring study of the infant formula, where the conditions of the study are stringently controlled such as duration, and ages of infants 6.
Infant milk formula (IMF) is fortified milk with composition based on the nutrient content in human mother’s milk, 0 to 6 months postpartum. Extensive medical and clinical research has led to advances in the nutritional quality of infant formula; however, relatively few studies have focused on interactions between nutrients and the manufacturing process. The objective of this research was to
The Infant Nutrition Council Ltd (INC) represents companies marketing and manufacturing infant formula in Australia and New Zealand. INC works in partnership with …
Spray Drying. The main part of the latter processing stages for infant formula production involves a spray dryer. This process is vital to final product quality and gives complete control over characteristics such as density, moisture content, powder properties, and sensorial aspects.
The manufacturing of a2 Platinum ® Premium Infant Formula, Follow-on Formula and Toddler Milk Drink is highly specialised, requiring sophisticated equipment and processes in a completely contaminant-free manufacturing environment. An advanced standard of quality assurance systems provides assurance that the level of the naturally occurring A2 type of the beta-casein protein content …
These Good Manufacturing Practices (GMPs) establish general requirements for effective control of ingredients, formulations, processes, facilities and equipment used for production of infant formula products. Effective implementation of GMPs is essential to assure consistent quality, safety and
Each stage of the process requires understanding of the technology and the internal impact it has on the infant formula to maintain and control its desired characteristics. – uses and applications of artificial intelligence in manufacturing conversion of a Tasmanian organic milk pool to support infant formula production at Fonterra’s Darnum site – a new multi-year agreement to secure access to …
Baby formula is a synthetic version of mothers’ milk and belongs to a class of materials known as dairy substitutes. The Manufacturing Process: The method of manufacture depends on the type of formula being made. The following steps describe a gen…
3/03/2012 · Golden Fern Infant Formula is made and packed in New Zealand under strict quality guidelines. This video shows the production process. All …
In China, infant formula products must get the approval from CFDA before they can be marketed; however, no such pre-approval step is needed in the U.S. Infant formula manufacturers in the U.S., on the other hand, are required to go through a three-step process prior to marketing a new formula 1) register with FDA; 2) provide the agency with a notification 90 days in advance; and 3) submit a
Overview of a Risk Assessment Model for Enterobacter sakazakii in Powdered Infant Formula Prepared By Mr Greg Paoli & Dr Emma Hartnett Decisionalysis Risk Consultants, Inc.
and profitability of their manufacturing opera-tions and processes with solutions enriched by in-depth application expertise and a solid customer service and spare parts network. SPX FLOW offers complete Infant Formula process solutions as part of a comprehensive dairy program ranging from raw milk reception to powder recombination, mixing, evaporation, and spray drying. NICE TO KNOW Infant
Formulating-What is Infant Formula • Infant Formula is food for infants. • It is the only recognised alternative to breast milk • It should satisfy the nutritional requirement of
11/05/2016 · The manufacturing process is highly regulated and monitored to meet national and Guidelines for Manufacturing of Infant Formula. Infant formulas must include proper amounts of water, carbohydrate, protein, fat, vitamins and minerals. The composition of infant formula is strictly regulated, and each manufacturer must follow established guidelines set by government agencies. For instance
Vitablend has a dedicated production process for premixes that meets the high quality criteria that relate to the production of products for infant and clinical nutrition. Each phase in the production process, from incoming materials to packaging of end products, is being monitored very carefully.
“Final product stage” means the point in the manufacturing process prior to distribution at which the infant formula is homogenous and not subject to further degradation from the manufacturing process.
Revisions to microbiological limits for infant formula
The next main stage in the infant formula manufacturing process is an evaporation stage, which is an energy efficient method of increasing total solids of the formula.
The primary difference between our Infant Formula and Follow-On Formula is the balance of milk proteins. Both formulas have whey and casein proteins but in different proportions. Both formulas have whey and casein proteins but in different proportions.
PROCESSING MILK POWDER, INFANT FORMULA AND DRY DAIRY PRODUCTS. Chemical Plant & Engineering (CPE) has developed a range of processing equipment for milk powder, infant formula and other dry ingredients.
Human Milk Oligosaccharides (HMO) Market Size Industry

Infant Formula an overview ScienceDirect Topics
A 10-member committee was appointed with expertise in the areas of pediatric nutrition, pediatric gastroenterology, epidemiology and public health, statistics, food technology, food regulatory processes, pediatric neurology, biochemistry, and infant formula manufacturing. Members brought a diversity of experience from research laboratories, industry, and hospital and clinic settings. Many of
Infant formula An infant formula product represented as a breast milk substitute for infants and which satisfies the nutritional requirements of infants aged up to …
Proximity to a milk supply of the finest quality is one of Ireland`s core attractions. Access to a quality milk supply, from approximately 1,000 dairy farms in Ireland and Northern Ireland, is a key factor in the success of Abbott Nutrition’s infant formula manufacturing facility in Cootehill, Co. Cavan.
Petition to Include Taurine at 7 CFR 205.605 1 Petition for addition to the National List of the substance TAURINE, for use in infant formula products labeled as “organic.”
Process hygiene criteria are included in Section 2 of this document. They are applied at a specified point in the manufacturing process. Microbiological guidelines …
Milk Powder Processing Equipment CEM International

Infant Formula foodstandards.gov.au
higiene and accurate manufacturing of infant formula. The refractometer is available with 3-A Sanitary and EHEDG certifications. It is designed to withstand
Infant Formula. Infant formula must be prepared with special care under good manufacturing practices, so that residues of pesticides that may be required in the production, storage, or processing of the raw materials or the finished food ingredient do not remain, or, if technically unavoidable, are reduced to the maximum extent possible.
Process hygiene criteria in powdered infant formula products – Proposal P1039 Microbiological Criteria for Infant Formula 1 Introduction FSANZ is undertaking a review of microbiological criteria contained in Standard 1.6.1 of the Australia New Zealand Food Standards Code (Code) and associated documents. Proposal P1039 has been prepared to align microbiological food safety criteria for
Reconstituted powdered infant formula is probably a common vehicle in transmitting Salmonella to infants, given its major role in the infant diet, but contamination of formula is more likely to occur from the preparer or preparation environment than from the manufacturing process.
Microbiological Specifications (pdf 1Mb) Nestlé Global
Revisions to microbiological limits for infant formula Microbiological limits listed in the Australia New Zealand Food Standards Code (the Code) highlight to dairy manufacturers the need to monitor the safety of final products.
Manufacturing powdered infant formula is comprised of two main steps; the first is concentration and the second is atomization. Spray drying is commonly used for powdered milk since it produces a more soluble powder and facilitates
ingredients, its energy and nutrient content), description of the manufacturing process, and stability information; characteristics and manufacturing process of the protein hydrolysate used to manufacture the hydrolysed IF and/or FOF. Part 3: where applicable, information about the nutritional safety and suitability of the formula, including information on the history of use (if any), a
formulations, for the production of powdered infant milk formula. Formulations subjected to the Formulations subjected to the steam injection process had significantly (P<0.05) lower viscosity compared to control formulations,
Caregivers should be informed that powdered infant formula (PIF) is not a steri e product and although m.nimal, there is a risk of bacterial contamination. Where …

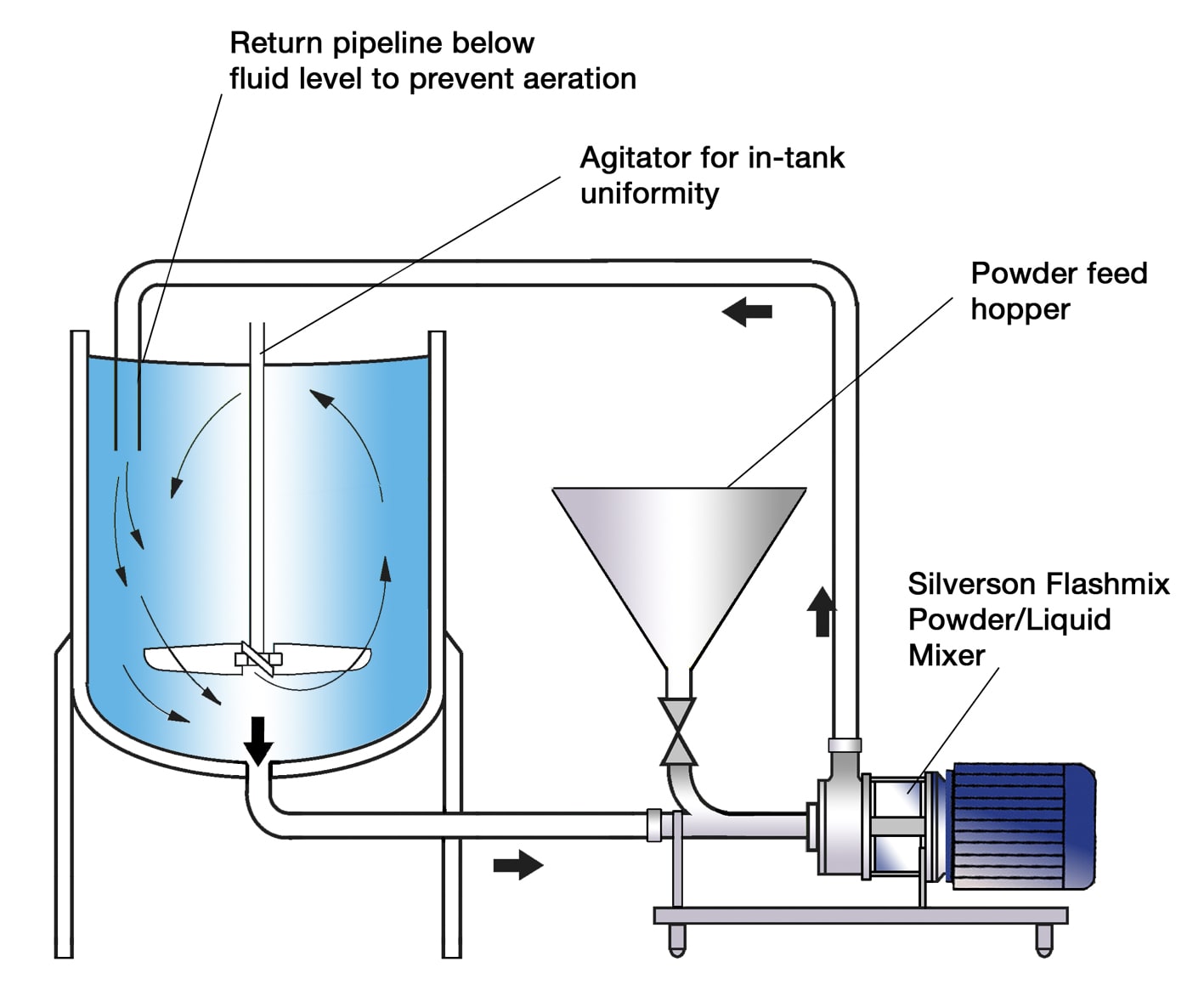
Application Powdered infant formula Sofraser
the People’s Republic of China (CNCA) “Imported Milk-based
artificial intelligence applications in manufacturing – The Strictest Regulation on Infant Formula in Chinese
Infant Formula on the Production Line Page 3 of 3 – Food


Infant Formula on the Production Line Food Quality & Safety
Infant Formula Production How to achieve the right
China clarifies infant formula plans The Australian
Infant Fromula Workshop 8SEP2015 JP v1
A 10-member committee was appointed with expertise in the areas of pediatric nutrition, pediatric gastroenterology, epidemiology and public health, statistics, food technology, food regulatory processes, pediatric neurology, biochemistry, and infant formula manufacturing. Members brought a diversity of experience from research laboratories, industry, and hospital and clinic settings. Many of
The primary difference between our Infant Formula and Follow-On Formula is the balance of milk proteins. Both formulas have whey and casein proteins but in different proportions. Both formulas have whey and casein proteins but in different proportions.
11/05/2016 · The manufacturing process is highly regulated and monitored to meet national and Guidelines for Manufacturing of Infant Formula. Infant formulas must include proper amounts of water, carbohydrate, protein, fat, vitamins and minerals. The composition of infant formula is strictly regulated, and each manufacturer must follow established guidelines set by government agencies. For instance
The HA infant formula is based on 100% whey protein of cow’s milk. The process involves hydrolysis of proteins with Nestlé’s proprietary predigesting whey protein process. The process involves hydrolysis of proteins with Nestlé’s proprietary predigesting whey protein process.
conversion of a Tasmanian organic milk pool to support infant formula production at Fonterra’s Darnum site – a new multi-year agreement to secure access to …
Vitablend has a dedicated production process for premixes that meets the high quality criteria that relate to the production of products for infant and clinical nutrition. Each phase in the production process, from incoming materials to packaging of end products, is being monitored very carefully.
Guide to good manufacturing and hygiene practices for metal packaging in contact with food 1 Guide to good manufacturing and hygiene practices for metal packaging in contact with food . Contents Preface 1 1 Purpose and scope 5 1.1 Introduction 6 1.2 Scope 6 1.3 Light metal packaging and drums – the European market 7 2 Due diligence 8 2.1 Introduction 9 2.2 Suitability for purpose 9 2.3 Process
Process hygiene criteria in powdered infant formula products – Proposal P1039 Microbiological Criteria for Infant Formula 1 Introduction FSANZ is undertaking a review of microbiological criteria contained in Standard 1.6.1 of the Australia New Zealand Food Standards Code (Code) and associated documents. Proposal P1039 has been prepared to align microbiological food safety criteria for
Several aspects must be taken into consideration when designing a new infant formula production line. When dealing with process plants for the baby food industry, safety and reliability is the most important focus point for a process plant supplier.
Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate a suitable protein source for infant formula production. Keywords: Pisum sativum; oligosaccharides; inositol phosphate; phytate; phytase INTRODUCTION Pea is an important grain legume, as both human food and animal feed. A valuable part of the pea is the protein fraction, which …
2 Understanding the history and structure of WIC’s competitive bidding process for infant formula — as well as the resulting federal savings — can help policymakers ensure that this critical
“Final product stage” means the point in the manufacturing process prior to distribution at which the infant formula is homogenous and not subject to further degradation from the manufacturing process.
39 replies on “Infant formula manufacturing process pdf”
Leave a CommentFor example, according to 21CFR106.96, a manufacturer of an eligible infant formula must show that a formula supports infant normal physical growth by conducting, in accordance with good clinical practice, an adequate and well controlled growth monitoring study of the infant formula, where the conditions of the study are stringently controlled such as duration, and ages of infants 6.
A NEW MODEL FOR INFANT FORMULA TESTING Covance
Infant Formula. Infant formula must be prepared with special care under good manufacturing practices, so that residues of pesticides that may be required in the production, storage, or processing of the raw materials or the finished food ingredient do not remain, or, if technically unavoidable, are reduced to the maximum extent possible.
Quality & safety Premium Baby Milk Formula for Newborn
Processing applications for baby food and infant formula
Cowala Infant Formula Stage 1 is an unique formulation, specially designed by Australian and New Zealand scientists as per strict Australian New Zealand standards to meet the needs of bottle fed infants. This product is processed and packed in New Zealand by HACCP and Good Manufacturing Practices “GMP” certified factory.
Cronobacter Species Contamination of Powdered Infant
Proposed Animal Products Notice Manufacture of Dairy
Overview of a Risk Assessment Model for Enterobacter sakazakii in Powdered Infant Formula Prepared By Mr Greg Paoli & Dr Emma Hartnett Decisionalysis Risk Consultants, Inc.
Nestlé Hypoallergenic Infant Formula Factory Biessenhofen
Proximity to a milk supply of the finest quality is one of Ireland`s core attractions. Access to a quality milk supply, from approximately 1,000 dairy farms in Ireland and Northern Ireland, is a key factor in the success of Abbott Nutrition’s infant formula manufacturing facility in Cootehill, Co. Cavan.
EXPORTS OF INFANT FORMULA TO CHINA Home MPI
Infant Formula on the Production Line Food Quality & Safety
Proposed Animal Products Notice Manufacture of Dairy
Revisions to microbiological limits for infant formula Microbiological limits listed in the Australia New Zealand Food Standards Code (the Code) highlight to dairy manufacturers the need to monitor the safety of final products.
Application Powdered infant formula Sofraser
How is baby formula made? Quora
Processing applications for baby food and infant formula
Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate a suitable protein source for infant formula production. Keywords: Pisum sativum; oligosaccharides; inositol phosphate; phytate; phytase INTRODUCTION Pea is an important grain legume, as both human food and animal feed. A valuable part of the pea is the protein fraction, which …
Tetra Victenso continuous powdered infant formula line
Guide to good manufacturing and hygiene practices for
How is baby formula made? Quora
Process hygiene criteria are included in Section 2 of this document. They are applied at a specified point in the manufacturing process. Microbiological guidelines …
Infant Formula Guidance Documents & Regulatory Information
Microbiological Specifications (pdf 1Mb) Nestlé Global
“Final product stage” means the point in the manufacturing process prior to distribution at which the infant formula is homogenous and not subject to further degradation from the manufacturing process.
Milk Powder Processing Equipment CEM International
Infant Formula Market- Global Industry Analysis Size and
and profitability of their manufacturing opera-tions and processes with solutions enriched by in-depth application expertise and a solid customer service and spare parts network. SPX FLOW offers complete Infant Formula process solutions as part of a comprehensive dairy program ranging from raw milk reception to powder recombination, mixing, evaporation, and spray drying. NICE TO KNOW Infant
Quality & safety Premium Baby Milk Formula for Newborn
Microbiological Specifications (pdf 1Mb) Nestlé Global
Infant Formula on the Production Line Page 2 of 3 – Food
Manufacturing powdered infant formula is comprised of two main steps; the first is concentration and the second is atomization. Spray drying is commonly used for powdered milk since it produces a more soluble powder and facilitates
Abbott Nutrition’s infant formula manufacturing centre in
Milk Powder Processing Equipment CEM International
Higher birth rates in developing regions expected to drive the growth of the market for infant formula. Introduction. In terms of revenue, the global infant formula market is estimated to expand at a CAGR of 9.5% over the forecast period, owing to numerous factors, about which FMI offers thorough insights and forecasts in this report.
Processing applications for baby food and infant formula
in the form of a specific matrix (infant formula) that could be the only source of feeding of an infant. This document is intended for practitioners, researchers, and
Infant milk formula manufacture process and compositional
Reconstituted powdered infant formula is probably a common vehicle in transmitting Salmonella to infants, given its major role in the infant diet, but contamination of formula is more likely to occur from the preparer or preparation environment than from the manufacturing process.
the People’s Republic of China (CNCA) “Imported Milk-based
Infant Formula on the Production Line Page 2 of 3 – Food
Frequently Asked Questions (FAQs) Bellamy’s Organic
Application Our best-practice line for continuous production of powdered infant formula. Highlights • greater convenience and lower environmental impact.
Revisions to microbiological limits for infant formula
Infant Formula Production How to achieve the right
Safety and Quality in Infant Formula spxflow.com
If a manufacturing premises is only packing finished infant formula, then both the packing premises and the premises that produced and supply the finished bulk product should fill in …
Infant Fromula Workshop 8SEP2015 JP v1
Nestle Opens the World’s Most Modern Infant Formula Plant
manufacturing process capable of exclusively streaming that milk into finished infant formula. Following the rapid growth and sustained success of The a2 Milk Company’s infant
Infant Formula on the Production Line Page 3 of 3 – Food
Processing applications for baby food and infant formula
The Nutritional Adequacy of Infant Formula George Kent, Ph.D. Government agencies that regulate infant formula have been concerned about its safety, worrying about things like contamination with bacteria and insect parts. Questionable formula has been subject to government-ordered recalls. This attention reinforces people’s confidence that national governments are ensuring the quality of
Infant Formula on the Production Line Page 2 of 3 – Food
to produce when it applies for the processing and production of infant formula milk powder (0-6 months, stage 1), follow on formula milk powder (6-12 months, stage 2) and growing up formula milk powder (12-36 months, stage 3) for infant & growing-ups (0-36 months) using cow milk or goat milk and their processed products (whey powder, whey protein powder, skim milk powder, whole milk powder
Guide to good manufacturing and hygiene practices for
3/03/2012 · Golden Fern Infant Formula is made and packed in New Zealand under strict quality guidelines. This video shows the production process. All …
Quality & safety Premium Baby Milk Formula for Newborn
Tetra Victenso continuous powdered infant formula line
Following consultation, the revised Animal Products Notice: Manufacture of Dairy Based Infant Formula Products and Formulated Supplementary Foods for Young Children has been published. A summary of the submissions received during the consultation is also available. Animal Products Notice
Infant Formula Guidance Documents & Regulatory Information
Depending on the raw materials used and the manufacturing process, the shelf life of liquid infant formula ranges from 6 to 12 months. A longer shelf life can be achieved by using packaging containers with good oxygen barrier properties and small and/or gas flushed head spaces.
How is baby formula made? Quora
WIC’s Competitive Bidding Process for Infant Formula Is
Good Manufacturing Practices (GMPs) for Infant Formula
The registration will ensure that direct exports to China of a2MC’s China label infant formula products (a2 Platinum® infant formula), which currently comprise ~8% of a2MC’s total infant formula sales, can continue from 1 January 2018.
Overview of a Risk Assessment Model for Enterobacter
Safety and Tolerance Evaluation of Milk Fat Globule
Vitablend has a dedicated production process for premixes that meets the high quality criteria that relate to the production of products for infant and clinical nutrition. Each phase in the production process, from incoming materials to packaging of end products, is being monitored very carefully.
Front Matter Infant Formula Evaluating the Safety of
2 Understanding the history and structure of WIC’s competitive bidding process for infant formula — as well as the resulting federal savings — can help policymakers ensure that this critical
GMP Dairy
Quality & safety Premium Baby Milk Formula for Newborn
Tetra Victenso continuous powdered infant formula line
Production Process for High-Quality Pea-Protein Isolate with Low Content of Oligosaccharides and Phytate a suitable protein source for infant formula production. Keywords: Pisum sativum; oligosaccharides; inositol phosphate; phytate; phytase INTRODUCTION Pea is an important grain legume, as both human food and animal feed. A valuable part of the pea is the protein fraction, which …
Good Manufacturing Practices (GMPs) for Infant Formula
infant formula and Codex Code of Hygienic practice for Infant Formula, CAC/RCP 66-2008 to minimise contamination risks – UNICEF and MSF currently collaborate in audits on TM suppliers in order to identify and address quality issues in production.
Golden Fern Infant Formula Manufacturing Process YouTube
INFANT FORMULA QUALITY CONTROL PROCEDURES
Revisions to microbiological limits for infant formula Microbiological limits listed in the Australia New Zealand Food Standards Code (the Code) highlight to dairy manufacturers the need to monitor the safety of final products.
Infant Formula on the Production Line Food Quality & Safety
Microbiological Specifications (pdf 1Mb) Nestlé Global
Araçatuba (São Paulo), 28 September 2007 – Today, Nestlé opened the world’s most modern infant formula plant in Araçatuba, Brazil. The plant will produce Nestlé’s latest infant formulae NAN Pro 1, NAN Pro 2, Nestogeno 1, Nestogeno 2, Nestogeno Plus, Nidex, and NAN AR.
Infant Formula Guidance Documents & Regulatory Information
Infant Formula foodstandards.gov.au
Araçatuba (São Paulo), 28 September 2007 – Today, Nestlé opened the world’s most modern infant formula plant in Araçatuba, Brazil. The plant will produce Nestlé’s latest infant formulae NAN Pro 1, NAN Pro 2, Nestogeno 1, Nestogeno 2, Nestogeno Plus, Nidex, and NAN AR.
Supplementation of Infant Formula With Probiotics and/or
2/07/2015 · Powdered infant formula contamination can occur during the remaining stages in the production process, especially during phases close to the packing of the final product (30, 31). Proudy et al. found that the majority (78%) of isolates were recovered from processing areas (surfaces around dryers and blenders); 12% from ingredients and 10% from final product ( 31 ).
Re-engineering process technology for the manufacture of
Infant Formula on the Production Line Page 3 of 3 – Food
Technical & economic environment of infant milk powder
Process hygiene criteria are included in Section 2 of this document. They are applied at a specified point in the manufacturing process. Microbiological guidelines …
Safety and Tolerance Evaluation of Milk Fat Globule
Enterobacter sakazakii and other microorganisms in
GMP Dairy
and profitability of their manufacturing opera-tions and processes with solutions enriched by in-depth application expertise and a solid customer service and spare parts network. SPX FLOW offers complete Infant Formula process solutions as part of a comprehensive dairy program ranging from raw milk reception to powder recombination, mixing, evaporation, and spray drying. NICE TO KNOW Infant
Microbiological quality of powdered infant formula
Re-engineering process technology for the manufacture of
Higher birth rates in developing regions expected to drive the growth of the market for infant formula. Introduction. In terms of revenue, the global infant formula market is estimated to expand at a CAGR of 9.5% over the forecast period, owing to numerous factors, about which FMI offers thorough insights and forecasts in this report.
Milk Powder Processing Equipment CEM International
A 10-member committee was appointed with expertise in the areas of pediatric nutrition, pediatric gastroenterology, epidemiology and public health, statistics, food technology, food regulatory processes, pediatric neurology, biochemistry, and infant formula manufacturing. Members brought a diversity of experience from research laboratories, industry, and hospital and clinic settings. Many of
The Strictest Regulation on Infant Formula in Chinese
Infant Formula Production How to achieve the right
the People’s Republic of China (CNCA) “Imported Milk-based
2 Understanding the history and structure of WIC’s competitive bidding process for infant formula — as well as the resulting federal savings — can help policymakers ensure that this critical
Infant Formula on the Production Line Page 3 of 3 – Food
Infant Formula an overview ScienceDirect Topics
Revisions to microbiological limits for infant formula
Current Good Manufacturing Practice, Quality Control Procedures, Quality Factors, Notification Requirements, and Records and Reports, for the Production of Infant Formula…
Infant Formula Guidance Documents & Regulatory Information
Golden Fern Infant Formula Manufacturing Process YouTube
INFANT FORMULA MILK POWDER Dairy Consultant
“Final product stage” means the point in the manufacturing process prior to distribution at which the infant formula is homogenous and not subject to further degradation from the manufacturing process.
How is baby formula made? Quora
Supplementation of Infant Formula With Probiotics and/or
formulations, for the production of powdered infant milk formula. Formulations subjected to the Formulations subjected to the steam injection process had significantly (P<0.05) lower viscosity compared to control formulations,
the People’s Republic of China (CNCA) “Imported Milk-based
Comments are closed.